 Armillaria
nabsnona, a new species from western North America
Armillaria
nabsnona, a new species from western North America
Center for Forest Mycology Research, Forest Products Laboratory, Forest Service, United States Department of Agriculture, One Gifford Pinchot Dr., Madison, Wisconsin 53705-23982
1Tom Volks current address: Dept. of Biology & Microbiology, 3024 Cowley Hall, University of Wisconsin-La Crosse, La Crosse, Wisconsin 54601 USA volk.thom@uwlax.edu
Originally appeared in Mycologia 88:484-491. 1996
Return to Tom Volk's Fungi Webpage http://TomVolkFungi.net
Abstract: Armillaria nabsnona is characterized morphologically and described as a new species. It is compatible with tester strains of North American biological species (NABS) IX. Using restriction fragment length polymorphism (RFLP) of the polymerase chain reaction (PCR) products amplified from the intergenic spacer (IGS) region of the ribosomal DNA, we also demonstrate an association of cultures of specimens with tester strains of NABS IX. Armillaria nabsnona appears to be restricted to several species of hardwood trees in the states of Washington, Oregon, California, Idaho and Alaska, and in the Canadian province of British Columbia. Basidiomata have been found in both the fall and spring.
Key Words: Agaricales, Basidiomycotina, NABS IX, biological species, systematics, Tricholomataceae.
INTRODUCTION
The genus Armillaria (Fr.:Fr.) Staude has undergone significant revision in the past twenty years. The genus once accommodated any white-spored agaric with broadly attached gills and an annulus (Volk & Burdsall, 1995). With Agaricus melleus Vahl:Fr. now accepted as the type species (Donk, 1962; Watling, Kile, and Gregory, 1982), Armillaria has a much narrower circumscription, including only those white-spored wood-inhabiting agarics with broadly attached to decurrent gills and forming macroscopic black to reddish-brown rhizomorphs.
Until the late 1970s Armillaria mellea (Vahl:Fr.) Kummer was considered by most researchers to be a pleiomorphic species with a wide host range and distribution. Herink (1973), among others, suspected that this single species might actually be a species complex. However, since basidioma morphology is difficult to study because of overlapping and apparently inconsistent traditional characters, other avenues of research were pursued. Hintikka (1973) developed a technique that allowed determination of mating types in Armillaria. Using a modification of this method, Korhonen (1978) was able to distinguish five European biological species (EBS). The technique depended on growing single spore isolates together in a petri dish and observing the change or lack of change in colony morphology. Single spore isolates of Armillaria species are generally white and fluffy, but when fusion of compatible mating types occurs, the coalesced colonies become dark brown, appressed, crustose, and sometimes produce rhizomorphs, depending on nutritional and cultural conditions. If the single spore isolates are from different species, the colonies will not grow together and will remain white and fluffy (Anderson and Ullrich, 1982). The five EBS have been characterized morphologically, and appropriate names have been given (see Watling, Kile, and Burdsall, 1991).
Anderson and Ullrich (1979) applied the techniques used by Korhonen to isolates collected from widely distributed locations in North America and demonstrated that what had been considered as Armillaria mellea in North America was actually 10 distinct biological species (North American biological species or NABS), some of which are compatible with EBS (Anderson et al., 1980; Anderson, 1986). Another, apparently rare, biological species, NABS XI, was recognized by Morrison et al. (1985). Three of the northeastern-occurring NABS were described and given names by Bérubé and Dessureault (1988, 1989). A tentative key to macromorphological identification of Armillaria species has been published (Burdsall & Volk, 1993). For additional nomenclatural details see Volk and Burdsall (1995).
The cumbersome nature of the mating type method of species identification has prompted a search for other techniques for identifying collections. Several methods have been employed with varying degrees of success including isozyme analysis (Morrison et al., 1985; Bérubé, 1994), cultural characteristics (Rishbeth, 1986), immunology (Burdsall and Banik, 1990) and molecular biological techniques (Harrington and Wingfield, 1995). Of these, molecular biological techniques seem to hold the most promise in terms of quick, accurate diagnosis from a variety of test materials. Harrington and Wingfield (1995) recently reported a technique involving restriction fragment length polymorphisms (RFLPs) of the ribosomal DNA intergenic spacer (IGS) region amplified by the polymerase chain reaction (PCR). They were able to separate all North American species of Armillaria except for A.calvescens and A.gallica, which are apparently very closely related (Anderson and Stasovski, 1992). In Harrington & Wingfield (1995), digestion of the IGS region of NABS IX with the restriction endonuclease Alu I yielded a unique banding pattern that distinguished it from the other species. This technique may prove invaluable for identifying collections of this and other species in the future, and thus we examined it in conjunction with the formal description of this species.
In spite of being a known entity, NABS IX has remained morphologically undescribed and not validly named. The species has been reported from Idaho (Anderson and Ullrich, 1979), California (Jacobs et al., 1994), Alaska (Shaw & Loopstra, 1988), and British Columbia (Morrison et al., 1985). The species has also been reported from Connecticut (Wargo, 1988), Newfoundland (Warren, 1994) and Japan (Mohammed et al., 1994), but these identifications are incorrect, as noted later in this work.
The major difficulty in naming and describing NABS IX has been finding specimens, and particularly, an appropriate type collection, partially because of the long lag period (up to two months) between collection of the basidiocarps and the determination of the NABS by mating studies. To resolve this problem, methodical collection, culture, and molecular studies were initiated in the fall of 1993 using large numbers of Armillaria basidiomata from the Olympic Peninsula of Washington. Identification was confirmed by compatibility with tester strains, and the morphological features of the basidiomata studied and characterized. The literature was also searched (Volk and Burdsall, 1995) to determine if there was an existing name for this fungus. Type specimens of northern temperate Armillaria species were also examined. No appropriate name was found, so the fungus is described here as a new species.
MATERIALS AND METHODS
Cultural and Morphological studies.-- Isolation of single basidiospores was made according to Darmono and Burdsall (1992) from fresh basidiomata collected from various areas on the Olympic Peninsula of Washington, USA, in October 1993. Compatibility with tester strains was determined according to the methods of Hintikka (1973), Korhonen (1978) and Anderson and Ullrich (1979) using second generation tester strains that we developed by testing against the original bank of tester strains of Anderson and Ullrich (1979). Specific strain information is available from the authors upon request. To determine sexuality of this species, pairwise crosses of ten single basidiospore isolates from TJV-93-188 were done on 1.5% malt extract, 2% agar medium (MEA). All cultures were incubated at 24 C.
Basidiomata were examined microscopically with phloxine in 3% KOH or in Melzer's solution. Colors in quotation marks are from Ridgway (1912). All specimens and cultures (single spore and tissue isolates) are deposited in the Center for Forest Mycology Research (CFMR) Madison, Wisconsin, USA, except where noted. Other herbarium abbreviations are taken from Holmgren et al. (1990).
Molecular studies.-- Eighteen tissue isolates of NABS IX and 2 single spore isolates of the original NABS IX testers (Anderson & Ullrich, 1979; Table I) were analyzed for RFLPs in the IGS region using the technique of Harrington and Wingfield (1995). Fungal material for PCR was obtained from mycelial mats grown on cellophane overlaying enriched medium (EM, Larsen et al., 1992). Approximately 25 mm2 of mycelial mat was ground with 500m L TE (10 mM Tris, pH 8, 1 mM EDTA) in a sterile 15 mL ground glass tissue homogenizer and centrifuged at 14000g for 10 min. The supernatant was diluted 1 to 100 in TE and 5m L of this was used as template DNA in PCR. The remaining PCR reaction mixture is as follows; 1.25 units Taq polymerase (Promega, Madison, WI), reaction buffer supplied with the enzyme (final concentrations 50 mM KCl, 12.5 mM Tris-HCl, pH-9, 0.1% Triton X-100), 1.5 mM MgCl , 200 uM each dNTP, and 0.2 uM of each primer in a volume of 25m L. The primers used were LR-12R (5' CTGAACGCCTCTAAGTCAGAA 3') and O-1 (5' AGTCCTATGGCCGTGGAT 3') (Operon Technologies, Alameda, CA). The reaction mixture was overlain with a drop of mineral oil. Thermocyler (Perkin-Elmer) parameters were as follows: One cycle of 93 C for 3 min, 53 C for 2 min and 72 C for 3 min followed by 29 cycles of 93 C for 1.5 min, 53 C for 2 min and 72 C for 3 min. This was followed by elongation at 72 C for 10 min. Following amplification, 3m L of the unpurified reaction product was digested for 16 h at 37 C with 5 U Alu I (GIBCO BRL, Gaithersburg, Maryland) mixed with the appropriate amount of buffer supplied with the enzyme in a total volume of 20m L. The digestion products were separated on a 4% agarose gel in tris-acetic acid-EDTA (TAE) buffer (pH 8) at 2.4 V/cm for 3-4 h. The products were visualized with UV illumination after being stained in an ethidium bromide solution for 10 min. The sizes of the digestion products were determined by comparing their migration distances to those of a 50-2000 bp standard (Biorad, Hercules, California) using a semi-logarithmic scale.
RESULTS
Mating studies.-- Single spore isolates from several collections of Armillaria from the Olympic Peninsula, which were "fluffy" in appearance, became appressed and dark when paired with tester strains of NABS IX. These new single-basidiospore isolates were paired with one another and exhibited a similar change in colony morphology, thus indicating they belong to a single compatibility group or biological species (Korhonen, 1978; Anderson & Ullrich 1979). Intra-basidioma matings between single spore isolates within three basidiomata indicated that the sexuality of this fungus is determined by two genetic loci; i.e. the fungus exhibits a tetrapolar mating system (data not shown).

| Table I. Armillaria nabsnona: Alu I restriction fragments of the amplified IGS region of 20 isolates. | ||
| Pattern Type | Isolates: | Fragment sizes (bp a) |
| a | Men-1, Men-2, Men-3, Shaw-CC, Am-111, 139-1, 121-1, OKM-25910, OKM- 25911, TJV-93-179 | 563 (552--575)
200 (144--206) |
| b | HB-18, HB-20, HB-21 | 306 (299--314)
230 (223--237) 196 (191--202) |
| c | TJV-93-166, TJV-93-173, TJV-93-177, TJV-93-188, TJV-93-198, TJV 93-200, M-90 | 560 (541--581)
321 (311--332) 237 (229--245) 203 (197--210) |
| a Fragment sizes based on comparison to DNA size standard. Values shown are based on an average of at least 12 different determinations from several gels. 95 percent confidence levels are indicated in parentheses. | ||
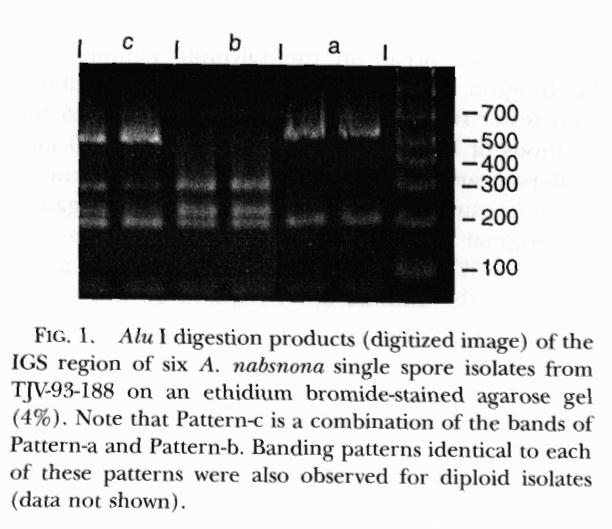
Molecular biology studies.-- The PCR product of the IGS region for each isolate was approximately 900 bp. Alu I digests of this product exhibited three different banding patterns (Fig. 1; Table I), two distinct patterns and a third that appeared to be a combination of the first two. Pattern-a consisted of 2 bands approximately 563 and 200 bp (similar in size to the 534 and 200 bp found for this species by actual counting of base pairs in the DNA sequences by Harrington and Wingfield, (1995) and was shared by 8 isolates and the single spore isolates of Anderson's testers. Pattern-b consisted of 3 bands approximately 306, 230 and 196 bp and was found in 3 isolates. Pattern-c consisted of bands approximately 560, 321, 237 and 203 bp and was present in 7 isolates. Bands smaller than 100 bp were not sized.
To determine if Pattern-c was a heterozygous condition resulting from the mating of Pattern-a and -b haplotypes, two Pattern-a and two Pattern-b single spore isolates were paired in all combinations. Each nonself pairing resulted in the development of a crustose type colony from which hyphal tips were microscopically excised from the last of three successive subcultures. Hyphal tip cultures that came from pairings of parents of like haplotypes exhibited the same Alu I digestion pattern as the parents. However, hyphal tip cultures that were obtained from the four pairings of parents with different haplotypes exhibited Alu I digest Pattern-c. Thus Pattern-c appears to be a heterozygous condition following a mating between haploids with Patterns-a and -b. In this respect the occurrence of Pattern-c indicates a compatible mating.
To determine the heritability of Pattern-c, the pattern types of 10 single spore isolates from a basidiome (TJV-93-188) with an associated Pattern-c tissue culture were determined. Six of the single spore isolates exhibited Pattern-a, two Pattern-b, and two Pattern-c.
DISCUSSION
The size of the amplification product and Alu I digestion Pattern-a of the IGS region were similar to that observed by Harrington and Wingfield (1995) for NABS IX. However, they did not observe Patterns-b and -c reported here. This is not surprising since they only examined haploid isolates from Anderson's original testers, which came from only two different fruiting bodies. None of the patterns observed here for the NABS IX isolates could be confused with the patterns observed for the other species of Armillaria reported by Harrington and Wingfield (1995). Pattern-b appears to be the result of an additional restriction site not present in Pattern-a. Thus the 563 bp fragment of Pattern-a is restricted once to yield the 306 and 230 bp fragments of Pattern-b.
The occurrence of Pattern-c in single spore isolates raises questions as to the true nature of the pattern. Since Pattern-b seems to result from the addition of a restriction site not present in Pattern-a isolates, it is conceivable that Pattern-c is actually an artifact of an incomplete Pattern-b digest. If this were the case, repeats of the digestions would yield inconsistent results with regard to the expression of either Pattern-c or -b. However, each isolate tested consistently exhibited only one pattern type when the Alu I digests were repeated.
Harrington (pers. comm.) has observed two pattern types in the IGS region of NABS IX isolates when digested with Hinc II. Upon digestion of our isolates with this enzyme we also found two restriction patterns that occurred in isolates with either Alu I Pattern-a or Pattern-b. The two Hinc II patterns also combined to make a third pattern in isolates that demonstrated Pattern-c with Alu I. Thus it appears that Pattern-c is not the result of partial digestion but of heterokaryon formation involving Pattern-a and -b haplotypes.
Interestingly, the two single spore isolates that showed Pattern-c with Alu I also showed a combined pattern with Hinc II. The presence of two different haplotypes in a single spore isolate could have several explanations. It is possible that the single-spore isolates actually contained more than one nucleus, either in a compatible or incompatible relationship, as has been proposed for other basidiomycetes (Kühner, 1977; Hallenberg, 1983). Morphologically these cultures appeared to be "typical" haploids, although one of the isolates displayed erratic mating behavior during the intrabasidiomata crosses. This may be an indication of the presence of two nuclei with hemicompatible mating types; Anderson and Ullrich (1982) found that auxotrophic haploid isolates of Armillaria could be paired to form common-A or common-B "fluffy" diploid prototrophs. Another less likely explanation for Pattern-c is that the IGS region may vary among its copies within a given nucleus. Despite this anomaly, for the NABS IX isolates tested, it appears that the Alu I digestion pattern of the IGS region is a reliable diagnostic tool.
Thus, two major lines of evidence point to the cultures and specimens we collected being contaxic with NABS IX. The single basidiospore isolates of the collected basidiocarps were compatible with tester strains of NABS IX and should be considered the same biological species. Three unique patterns of digest with Alu I also link the specimens and cultures with tester strains of NABS IX. With basidiomata in hand, we are able to describe a new species of Armillaria with distinctive characteristics.
DESCRIPTION OF SPECIES
Figs. 2-4. Armillaria nabsnona holotype, TJV-93-188. 2. Basidiomata 3. Pileus surface. 4. Habit. Note gregarious nature of the basidiomata. Click on any of the pictures to view larger (about 200kb) images
Armillaria nabsnona Volk & Burdsall sp. nov. Figs. 2-9
Fungiformi, pileo 4-5 cm diam, aurantio-brunneo; lamellis adnatis vel decurrentibus; stipite 8-10 cm X 2-5 mm, brunneo; velo supero, affixo 2-3 cm ab apice; contextu 0.5-1 mm lato; basidiis 25-35 x 5.5-6 m m, nodose-septatis; basidiosporis ovoideis vel subglobosis, (6-) 8-10 X 5.5-6.5 m m, inamyloideis; habitatio riparum, gregario ad lignum angiospermarum arborum.
HOLOTYPE. TJV-93-188 on fallen trunk of Acer macrophyllum Pursh, Hoh River Rain Forest Trail, Olympic National Park, Jefferson County, Washington, USA, leg. Thomas J. Volk, 19 October 1993; deposited CFMR. Type culture: tissue and single spore isolates, deposited CFMR.
Etymology. Named for NABS IX: nabs = acronym for North American biological species, nona = ninth.
Basidiomata tricholomatoid (Fig. 2-4). Pileus at first convex, later plane; 4-7 cm broad when fully expanded; surface smooth, hygrophanous, slimy-appearing when wet (Fig. 3), sometimes with short dark fibrils ("hairs") on the disc when young; disc "snuff brown," paler toward margins, often with darker irregular bruise-like areas on or near the surface; margin slightly incurved, "clay color," translucent striate (striate appearance due to observation of gills through the thin flesh) to furrowed. Context 0.5-1 mm thick, white. Lamellae adnate to subdecurrent, subdistant, 0.75-1 mm wide, white to cream colored, darkening in age to pinkish-tan, often developing brownish patches. Stipe 8-10 cm long, 4-5 mm broad at base, narrowing to 2-3 mm broad at apex, "mummy brown" at base, paler "buckthorn brown" to "warm buff" at the annulus; with white cottony patches below the annulus; context white, fibrous, "peeling" away in parallel strips (as is typical for Armillaria species). Partial Veil in buttons dense white cottony until rupture; annulus flaring upward at first (Fig. 4), soon becoming ragged as the pileus expands, sometimes persisting as an evanescent cortina, but more often washed away and difficult to observe, sometimes completely lost upon drying. Rhizomorphs frequently lacking; when present, thick (1-2 mm broad), black, and branching.
 Figs.
5-9. Armillaria nabsnona holotype, TJV-93-188. Black bar = 10 m
m. 5. Basidiospores. 6. Mature basidia. 7. Young basidia showing pattern
of branching. Note that the second basidium arises from the clamp of the
first; and the third basidium from the clamp of the second. 8. Pileus cuticle
cross section. 9. Clamped and unclamped septa of cells of the subhymenium.
Figs.
5-9. Armillaria nabsnona holotype, TJV-93-188. Black bar = 10 m
m. 5. Basidiospores. 6. Mature basidia. 7. Young basidia showing pattern
of branching. Note that the second basidium arises from the clamp of the
first; and the third basidium from the clamp of the second. 8. Pileus cuticle
cross section. 9. Clamped and unclamped septa of cells of the subhymenium.
Basidiospores white in mass; ovoid to subglobose; (6-)8-10 X 5.5-6.5 m m, smooth, somewhat thick-walled at maturity, hyaline, negative in Melzer's Reagent (Fig. 5). Basidia clavate; 25-35 X 5.5-6 m m, 3-4 m m broad just distal to clamped basal septum; four-sterigmate; sterigmata 4.5-6 m m long at maturity, 1 m m wide at base; with a clamp connection at base (Fig. 6); a second basidium usually forms from the basal clamp connection, sometimes with a third basidium developing from a clamp on the second (Fig. 7). This is most obvious in young incompletely expanded basidiomata. Cystidia not observed, but thin (2-3 um) wide hypha-like cells can sometimes be found among basidia. Pileipellis composed of swollen unbranched, hyaline, terminal cells, 40-60 X 14-16 um; with an underlying layer of more or less parallel, hyaline hyphae, 7-8 m m wide (Fig. 8). Partial Veil composed of more or less parallel hyaline hyphae, 4-7 m m diam, rarely branching, with frequent simple septa. Stipe Context composed of parallel and infrequently branching, simple septate, hyaline hyphae; elements 10-15 m m wide, sometimes swollen in the mid-region. Stipitipellis of closely appressed, parallel, simple-septate, brown hyphae that frequently anastomose with H-connections between the parallel hyphae; elements 8-10 m m broad, not swollen. Subhymenium with large clamp connections present on some hyphae (Fig. 9) especially on the 2-3 septa proximal to the basidia where hyphal branches proliferate directly from the clamps. Gill trama regular, hyphae usually simple septate, with occasional clamp connections.
Habit and Habitat. Gregarious, but not caespitose (Fig. 4), forming from branched black rhizomorphs or from 1 mm thick pseudosclerotial plates in the wood. Basidiomata have been found in both the fall (Sept.-Nov.) and the spring (April in Oregon).
Specimens Examined.--(* indicates cultures also studied and on deposit): USA, WASHINGTON: TJV-93-188 (holotype) on fallen trunk of Acer macrophyllum Pursh, Hoh River Rain Forest Trail, Olympic National Park (ONP), Jefferson County, leg. T.J. Volk, 19 Oct. 1993. CFMR*; TJV-93-200, on trunk of Acer circinatum Pursh, Hoh River Rain Forest, 200 m west of Rain Forest Trail, ONP, Jefferson County, G.R. Walker Experimental Transect 10-4-93HO, Plot 7, leg. G.Walker and M.Puccio, 20 Oct. 1993. CFMR*; TJV- 93-198, on down log of Alnus rubra Bong., flood plain of Hoh River, ONP, Jefferson County, leg. T.J. Volk, 20 Oct. 1993, CFMR*; OKM-25910, on Alnus sp. under very large Picea sitchensis and Tsuga heterophylla. Klahanie campground, Olympic National Forest, Clallam County, leg. O.K. & H.Miller, L. & M.Bailey, 14 Oct. 1993. CFMR*, VPI*; OKM- 25911, Alnus rubra snag, Klahanie campground, Olympic National Forest, Clallam County, leg. M. & L.Bailey, O.K. & H.Miller. 14 Oct. 1993. CFMR* VPI*; USA, CALIFORNIA: Men-39,(=Men 1, specimen 39, = UC 1598524) soil, Mendocino County, leg. K.Jacobs, (Jacobs et al., 1994) Spring 1990, CFMR. UC.; USA, OREGON: JR-A-1, JR-A-2, JR-A-3, JR-A-4, Rivermill Park, Estacada, Clackamas Co., Oregon Elev. 400 ft. on Acer circinatum roots and buried limbs, leg. J.A. Roger 14-21 April 1995, CFMR*; CANADA, BRITISH COLUMBIA: HHB-13535, Alnus sp. log, in mixed conifer area, One Mile Creek, near Callaghan Lake, Garibaldi National Forest, near Whistler BC. leg. H.H. Burdsall, Jr., 6 Oct. 1990. no cultures; DAVFP-24864, Acer macrophyllum, Goldstream, Vancouver Island, leg. D.Morrison, 20 Oct. 1990. 94-9-5003-01. CFMR, DAVFP; DAVFP- 24863, Alnus rubra, Goldstream, Vancouver Island, leg. D.Morrison, 20 Oct. 1990. 94-9-5003-01. CFMR, DAVFP.
Other Cultures Studied.--To provide additional information on hosts and geographical distribution the following additional cultures were studied.
Other cultures, without known specimens, collected and determined by Duncan J. Morrison as NABS IX and deposited at DAVFP (Morrison, pers. comm.) CANADA, BRITISH COLUMBIA: Sp8128, Parksville, Vancouver Island, unknown host, Oct. 1981; Sp8130, Sp8131, Ladysmith, Vancouver Island, Alnus rubra, Oct. 1981; Sp8132, Colwood, Vancouver Island, Acer macrophyllum, Oct. 1981; Sp8133, Colwood, Vancouver Island, Alnus rubra, Oct. 1981; Sp8135, Egmont, unknown host, Oct. 1981; Sp8258, Squamish BC, Populus trichocarpa Torr. & Gray, 4 Nov. 1982; Sp8260, Squamish, Alnus rubra, 4 Nov. 1982; Sp8261, Vancouver, Univ. of BC campus, Alnus rubra, 4 Nov. 1982; Sp8263, Vancouver, unknown host, 4 Nov. 1982; Sp8352, Hope, Alnus rubra, 27 Oct. 1983; Sp8361, Lake Cowichan, Vancouver Island, Alnus rubra, 1 Nov. 1983.
Other cultures at CFMR without known specimens. CANADA, BRITISH COLUMBIA: 121-1, 121-2, Acer macrophyllum, Anderson et al., 1980, AM-111, Morrison, Coquihalla BC; USA, IDAHO: 139-1, 139-2, unknown host, Anderson et al., 1980; USA, CALIFORNIA: Alnus rubra, Anderson and Ullrich, 1979; USA, IDAHO: HB-19, McDonald, 1992, Priest River Experimental Forest, rotten log leg. G. McDonald; HB-20, 1992, Priest River Experimental Forest, Idaho, leg. G. McDonald; HB-21, 1992, Priest River Experimental Forest, Idaho, Elderberry, leg. G. McDonald; USA, ALASKA: Arm.- Shaw C.C., 5 Nov. 1989, Juneau, Alaska, leg. G.C. Shaw.
Comments.--Armillaria nabsnona can be identified most easily by its geographical distribution, primarily the west coast of North America, and host range, primarily on hardwoods in riparian areas, especially frequent on Alnus species. Macroscopic characters that may be used to distinguish A.nabsnona from other North American species include a more orange coloration when fresh and also a narrower stipe in comparison to the size of the pileus. The stipe is darker than other Armillaria species, especially when dried. There are no scales, but small black hairs may be present on the surface of the pileus, a similar situation to that found in A. mellea. Microscopically, Armillaria nabsnona can be distinguished from other western species of Armillaria by the pattern of branching of the basidia; the second basidium emerges from the clamp of the first, the third basidium emerging from the clamp of the second, and so on. This is particularly obvious in immature specimens, but often becomes obscured in more mature specimens as the basidia become larger and more closely packed together. Armillaria nabsnona has unbranched terminal cells in the cuticle of the pileus, distinguishing it from the similar A. mellea, which has frequently-branching terminal cells in the pileus cuticle. Armillaria mellea also lacks clamps at the bases of the basidia. Although it fruits much more commonly in the fall, Armillaria nabsnona is also the only Armillaria species so far found fruiting in the spring in the Pacific Northwest, although the frequency of this phenomenon is not known.
The Armillaria cultures from Connecticut on conifer (Wargo, 1988) and on black oak (Strain AW-20; Wargo, pers. comm.), indicated as NABS IX by Wargo and mentioned by Burdsall and Volk (1993) have been retested and are not compatible with tester strains of NABS IX or with any other biological species (Wargo, pers. comm.), although Harrington (pers. comm.) reports that strain AW-20 shows the RFLP pattern of Armillaria ostoyae (Romagn.) Herink (NABS I).
Warren (1994) stated that NABS IX is the most prevalent species in Newfoundland, but Bérubé (1994) found only A.ostoyae in his isozyme study of 39 Newfoundland isolates, many of which were Warren's (Warren and Bérubé, pers. comm.). Thus, Armillaria nabsnona has not been confirmed in North America east of the Rocky Mountains.
Guillaumin et al. (1988), Kile et al. (1994) and Mohammed et al. (1994) have stated that NABS IX has been isolated from the Japanese island of Hokkaido. J. J. Guillaumin generously provided these isolates to us; we retested them in "dip-hap" crosses and found no evidence for compatibility of the Japanese isolates with our fresh NABS IX testers. In their study of the Armillaria species on Hokkaido, Cha et al. (1994; Cha, pers. comm.) have also reported finding none of their Armillaria isolates compatible with testers of NABS IX. There is no good evidence, in our opinion, that NABS IX exists in Japan or anywhere outside Northwestern North America.
ACKNOWLEDGMENTS
We thank O. K. Miller (VPI) for gift of specimens, slides, and descriptions; W. J. Sundberg (SIU), D. M. Rizzo (CFMR, UCD), and T. C. Harrington (ISU) for review of manuscript; J. Ammirati (WTU) for support of collecting; J. Paul (CFMR) for technical assistance; K. K. Nakasone (CFMR) for help with the Latin diagnosis; D. J. Morrison and B. Callan (DAVFP) for generous gift of specimens and information on cultures; K. Jacobs (Morton Arboretum, Illinois) and J. J. Guillaumin (INRA, France) for gift of cultures; G. Walker and M. Puccio (WTU), H. Miller, M. Bailey, and L. Bailey for assistance in collecting; P. M. Wargo, (Hamden, CT), G. McDonald (Moscow, ID), G. C. Shaw (Fort Collins, CO), and G. Kile (CSIRO, Australia) for information on distribution; I. Tavarres (UC) for loan of specimens; M. J. Larsen (Moscow, ID) for helpful comments: and Judy Roger (Estacada, OR) for collection and gift of spring-fruiting specimens. We also thank the Olympic National Park and Olympic National Forest for permission to collect within their boundaries.
LITERATURE CITED
Anderson, J. B. 1986. Biological species of Armillaria in North America: redesignation of groups IV and VIII and enumeration of voucher strains for other groups. Mycologia 78: 837-839.
----, K. Korhonen, and R. C. Ullrich. 1980. Relationships between European and North American biological species of Armillaria mellea. Exper. Mycol. 4: 87-95.
----, and E. Stasovski. 1992. Molecular phylogeny of Northern Hemisphere species of Armillaria. Mycologia 84: 505-516.
----, and R. C. Ullrich. 1979. Biological species of Armillaria in North America. Mycologia 71: 402-414.
----, and ----. 1982. Diploids of Armillaria mellea: synthesis, stability, and mating behavior. Canad. J. Bot. 60: 432-439.
Bérubé, J. A. 1994. Identification of Newfoundland Armillaria species using isoenzymes. Pp. 355-357. In: Proceedings of the eighth IUFRO conference on root and butt rots. Eds. M. Johansson and J. Stenlid. Uppsala: SLU Info/Repro.
----, and M. Dessureault. 1988. Morphological characterization of Armillaria ostoyae and Armillaria sinapina sp. nov. Canad. J. Bot. 66: 2027-2034.
----, and ----. 1989. Morphological studies of the Armillaria mellea complex: Two new species, A. gemina and A. calvescens. Mycologia 81: 216-225.
Burdsall, H. H., Jr. and M. T. Banik. 1990. Serological differentiation of three species of Armillaria and Lentinula edodes by enzyme-linked immunosorbent assay using immunized chicken as a source of antibodies. Mycologia 82: 415-423.
Burdsall, H. H., Jr. and T. J. Volk. 1993. The state of taxonomy of the genus Armillaria. McIlvainea 11: 4-12.
Cha, J. Y, J. M. Sung and T. Igarashi. 1994. Biological species and morphological characteristics of Armillaria mellea complex in Hokkaido: A. sinapina and two new species, A. jezoensis and A. singula. Mycoscience 35: 39-47.
Darmono, T. W. and H. H. Burdsall, Jr. 1992. Morphological characterization of incompatibility reactions and evidence for nuclear migration in Armillaria mellea. Mycologia 84: 367-375.
Donk, M. A. 1962. The generic names proposed for the Agaricaceae. Beih. Nova Hedwigia 5: 1-320.
Guillaumin, J. J., C. Mohammed, and S. Berthelay. 1988. Armillaria species in the northern temperate hemisphere. Pp. 27-43 In: Proceedings of the Seventh IUFRO Conference on Root and Butt Rots; Ed. D.J. Morrison. Forestry Canada, Victoria, British Columbia, Canada.
Hallenberg, N. 1983. Hericium coralloides and H.alpestre (Basidiomycetes) in Europe. Mycotaxon 18: 181-189.
Harrington, T. C. and B. D. Wingfield. 1995. A PCR-based identification method for species of Armillaria. Mycologia 87: 280-288.
Herink, J. 1973. Taxonomie Václavky Obecné- Armillaria mellea (Vahl. ex Fr.) Kumm. Pp. 21-48. In: Vysoká Skola Zem_d_lska V Brné. Vyznamenaná Rádem Prace BRNO; J. Hasek, ed. Brno: Lesnicka fakulta VSZ.
Hintikka, V. 1973. A note on the polarity of Armillariella mellea. Karstenia 13: 32-39.
Holmgren, P. K., N. H. Holmgren, and L. B. Barnett. 1990. Index Herbariorum. Eighth Edition. New York Botanical Garden, New York. 693 pp.
Jacobs, K., J. D. MacDonald, F. W. Cobb, Jr., and K. Wells. 1994. Identification of Armillaria species in California. Mycologia 86: 113-116.
Kile, G. A., J. J. Guillaumin, C. Mohammed, and R. Watling. 1994. Biogeography and Pathology of Armillaria. Pp. 411-436. In: Proceedings of the eighth IUFRO conference on root and butt rots. Eds. M. Johansson and J. Stenlid. Uppsala: SLU Info/Repro.
Korhonen, K. 1978. Interfertility and clonal size in the Armillaria mellea complex. Karstenia 18: 31-42.
Kühner, R. 1977. Variation of nuclear behaviour in the homobasidiomycetes. Trans. Brit. Mycol. Soc. 68: 1-16.
Larsen, M. J., M. T. Banik, and H. H. Burdsall, Jr. 1992. Clamp connections in North American Armillaria species: occurrence and potential application for delimiting species. Mycologia 84: 214-218.
Mohammed, C., J. J. Guillaumin, and S. Berthelay. 1994. Armillaria species identified in China and Japan. Mycol. Res. 98: 607-613.
Morrison, D. J., D. Chu, and A. L. S. Johnson. 1985. Species of Armillaria in British Columbia. Canad. J. Plant Pathol. 7: 242-246.
Ridgway, R. 1912. Color Standards and Color Nomenclature. 53 plates.
Rishbeth, J. 1986. Some characteristics of English Armillaria in culture. Trans. Brit. Mycol. Soc. 85: 213-218.
Shaw, C. G. III, and E. M. Loopstra. 1988. Identification and pathogenicity of some Alaskan isolates of Armillaria. Phytopathology 78: 971-974.
Volk, T. J. and H. H. Burdsall, Jr. 1995. A nomenclatural study of Armillaria and Armillariella species. Synopsis Fungorum 8. Fungiflora: Oslo, Norway. 121 pp.
Wargo, P. M. 1988. Elevation and Armillaria species relationships in spruce-fir forests of northeastern United States. Pp. 340-346. In: Proceedings of the Seventh IUFRO Conference on Root and Butt Rots; Ed. D.J. Morrison. Forestry Canada, Victoria, British Columbia, Canada.
Warren, G. R. 1994. The Armillaria species complex in Newfoundland. Pp. 368-375. In: Proceedings of the eighth IUFRO conference on root and butt rots. Eds. M. Johansson and J. Stenlid. Uppsala: SLU Info/Repro.
Watling, R., G. A. Kile and N. M. Gregory. 1982. The genus Armillaria - Nomenclature, typification, the identity of Armillaria mellea and species differentiation. Trans. Brit. Mycol. Soc. 78: 271-285.
----, ----, and H. H. Burdsall, Jr. 1991. Nomenclature, Taxonomy and Identification. Pp. 1-9. In: Armillaria Root Disease, C.G. Shaw and G. Kile, eds. Agriculture Handbook No. 691. USDA Forest Service. Washington, D.C.