
Thomas J. Volk, Dept. of Biology , 3012 Cowley Hall, University of Wisconsin- La Crosse, La Crosse WI 54601 volk.thom@uwlax.edu
Return to Tom Volk's Fungi Home Page
See also this page, where the morel was Fungus of the Month.

Modified from an article originally published in McIlvainea 10(1):76-81, 1991. as "Understanding the morel life cycle: Key to cultivation"

The morel, one of the most prized and most delicious mushrooms, has finally been cultivated. The morel is perhaps the best known of the edible fungi, since it is easily identified even by those who have but a slight knowledge of mycology, one of Christiansen's Infallible Four. Although many "lesser" mushrooms are easily cultivated, e.g. Agaricus brunnescens (= A. bisporus), Pleurotus spp., and shiitake, the morel has defied all attempts at consitent indoor cultivation until very recently.
Here in the Midwest, one of the major pleasures of the spring season is going out in the woods after the snow melts to see the variety of ephemeral plants and fungi that are not present at other times of the year because of shading by large deciduous trees. Almost as an added bonus, it is possible that if one finds some dead elm trees, old apple orchards, some black cherry trees, or the results of a previous year's fire, and if there has been enough rain and if the temperature is neither to hot nor too cold and if the winter has been neither too mild nor too severe, and if one happens to be at the right place at the right time, one may also find morels. It is not easy to predict in advance of a hunt whether there will be some morels found in a particular place, even in a known "morel spot." The thrill of the hunt is precisely what makes morelling so exciting... and often so frustrating. Nearly every morel hunter has a ready list of excuses why no morels are being found: too hot, too cold, not enough rain, too much rain, not enough humidity, too humid, the tree hasn't been dead long enough, the tree's been dead too long, the may apples aren't blooming yet, the oak leaves aren't yet the size of a squirrel's ear, and so on. The apparent lack of identifiable consistent conditions that lead to wild morel fruiting has been a major deterrent in establishing protocols for artificial morel cultivation.
 Almost as if to further confound the facts, morels fruit in the western
United States in a much different manner. They rarely have any relationship
with particular plants or trees, but instead appear most often in a variety of
disturbed habitats, especially after forest fires, in logging "skid trails,"
and in horticultural plantings, especially in "bark dust." A trip in 1991 to
the Malheur National Forest in eastern Oregon revealed morels coming up in
places and under conditions I would not even consider looking in the midwest
or east. On the site of a massive forest fire the previous year, we saw
literally hundreds of morels within a few hundred square feet, and the fire
covered several hundred acres, so one can imagine the large number of morels
during the entire fruiting season. The snow had melted about three weeks
earlier at the site we visited, and, although the ground appeared to be quite
dry, the morels were appearing everywhere, especially on the sides of the
holes created where trees burned well into the ground. According to area
foresters, there had not been morels on this site in any previous years,
probably not since fires ravaged the area 100 years previously. The
ecological aspects of the situation, e.g. the role of morel mycelium in the
healthy forest, is not yet clear and is often confusing and frustrating for
those of us looking for a consistent pattern.
Almost as if to further confound the facts, morels fruit in the western
United States in a much different manner. They rarely have any relationship
with particular plants or trees, but instead appear most often in a variety of
disturbed habitats, especially after forest fires, in logging "skid trails,"
and in horticultural plantings, especially in "bark dust." A trip in 1991 to
the Malheur National Forest in eastern Oregon revealed morels coming up in
places and under conditions I would not even consider looking in the midwest
or east. On the site of a massive forest fire the previous year, we saw
literally hundreds of morels within a few hundred square feet, and the fire
covered several hundred acres, so one can imagine the large number of morels
during the entire fruiting season. The snow had melted about three weeks
earlier at the site we visited, and, although the ground appeared to be quite
dry, the morels were appearing everywhere, especially on the sides of the
holes created where trees burned well into the ground. According to area
foresters, there had not been morels on this site in any previous years,
probably not since fires ravaged the area 100 years previously. The
ecological aspects of the situation, e.g. the role of morel mycelium in the
healthy forest, is not yet clear and is often confusing and frustrating for
those of us looking for a consistent pattern.
 It is precisely this frustration and overall lack of knowledge as well as
the general "mystique" that envelopes the morel, that has generated the
excitement of the patenting of a process to grow morels (Morchella sp.) under controlled conditions (U.S. Patent nos. 4,594,809 and 4,757,640). Most people
ask the same few questions: does the process really work? Is the process
repeatable and consistent? Why are morels so difficult to fruit under
controlled conditions? If mushroom growers can get Agaricus, shiitake and
oysters mushrooms to form, why should the morel be different? The answers to
these questions lie in an understanding of the life cycle of the morel.
It is precisely this frustration and overall lack of knowledge as well as
the general "mystique" that envelopes the morel, that has generated the
excitement of the patenting of a process to grow morels (Morchella sp.) under controlled conditions (U.S. Patent nos. 4,594,809 and 4,757,640). Most people
ask the same few questions: does the process really work? Is the process
repeatable and consistent? Why are morels so difficult to fruit under
controlled conditions? If mushroom growers can get Agaricus, shiitake and
oysters mushrooms to form, why should the morel be different? The answers to
these questions lie in an understanding of the life cycle of the morel.
 Figure 1 is a representation of the morel life cycle (Volk and Leonard
1990, Volk and Leonard, 1989a). Even a glance at the figure will reveal a
stage of the morel life cycle not present in the other cultivated mushrooms:
the sclerotium. The sclerotium of the morel is a relatively large structure
(1mm -5 cm diameter) composed of large cells with very thick walls that allow
the fungus to survive adverse natural conditions, such as winter. In the
spring, the sclerotium has two options for germination; to form a new mycelium
or to form a fruiting body. Unfortunately for the potential grower, it is
very easy to get the sclerotia to form a new mycelium but very difficult to
force it to form a fruiting body. Very specific conditions of nutrition,
humidity, carbon dioxide levels and temperature must be met for primordia to
form. Although difficult, however, this part is relatively easy. The
primordium is very prone to abort at this very young stage if not given the
proper set of conditions, which may be very different from those that allowed
their initiation. It is at best a very tricky business.
Figure 1 is a representation of the morel life cycle (Volk and Leonard
1990, Volk and Leonard, 1989a). Even a glance at the figure will reveal a
stage of the morel life cycle not present in the other cultivated mushrooms:
the sclerotium. The sclerotium of the morel is a relatively large structure
(1mm -5 cm diameter) composed of large cells with very thick walls that allow
the fungus to survive adverse natural conditions, such as winter. In the
spring, the sclerotium has two options for germination; to form a new mycelium
or to form a fruiting body. Unfortunately for the potential grower, it is
very easy to get the sclerotia to form a new mycelium but very difficult to
force it to form a fruiting body. Very specific conditions of nutrition,
humidity, carbon dioxide levels and temperature must be met for primordia to
form. Although difficult, however, this part is relatively easy. The
primordium is very prone to abort at this very young stage if not given the
proper set of conditions, which may be very different from those that allowed
their initiation. It is at best a very tricky business.
Attempts have been made for many years to cultivate morels. The high price that is commanded by these delicacies ($15-$20 per pound at farmers' markets in Wisconsin--even with a plentiful crop) has tempted many mushroom growers; the out-of-season price for morels could certainly be much higher than this. There are scientific reports on success with growing morels from as early as 1883 (using such things as jerusalem artichokes, apples, pumpkin and various other substrates), and certainly there must have been attempts at cultivation long before that. Many people (myself included) have had success with fruiting morels in their backyards by simply throwing out the wash water from collected morels into their compost piles, their lawn, or (in my case) under some volunteer elm saplings growing up against the house. The following spring, morels may appear, sometimes in great abundance. There are kits available commercially that serve the same purpose. Yes they do really work, but backyard success is often a matter of luck, although there are several tricks that may be employed (such as "proper" burning, frequent watering, or killing the elm saplings at the right time of year) to greatly enhance one's success rate.
Some possible reasons for difficulty in fruiting the morel are made very clear by examination of the time frame involved with fruiting of fire-induced western morels. A massive fruiting of morels occurs after a forest fire. Many spores are released that eventually germinate to form mycelia and then form sclerotia before the next snow cover. They do not fruit again, existing in the soil in the form of sclerotia or cycling between the sclerotial and mycelial stages, until the next forest fire, on the average 80-100 years later. Given this time frame and our lack of knowledge concerning the natural role of the morel in this ecosystem, it is not surprising that artificial cultivation of morels, even outdoors, is so difficult.
Despite some scattered reports of outdoor cultivation, however, attempts to control fruiting indoors met with only scattered success until Ronald Ower of San Francisco succeeded in regularly cultivating morels, publishing his results in 1982. Naturally, the methods he described in his paper were quite vague. (After all, if you discovered something that important, you would want everyone to know about it, but you wouldn't want them to be able to do it also!) Ower had difficulty convincing any established mushroom company that he had a process that worked and that the company would be able to make a profit. At this point the Neogen company of East Lansing, Michigan became interested in the process. Neogen is affiliated with Michigan State University and at that time was already the holder of a number of biotechnology patents. Neogen was able to convince Ower to come to East Lansing to develop a commercial process for growing morels. In April of 1986, U.S. Patent no. 4,594,809 was issued. Tragically, Ron Ower did not live to see the patent granted; he had been murdered a few weeks before in San Francisco.
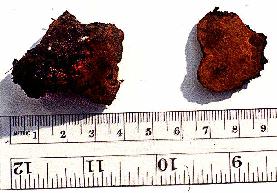 The patents themselves (see also Volk and Leonard, 1989b) describe a
process for formation of sclerotia that are competent to produce fruiting
bodies. Morel sclerotia do not normally form until the nutrients of a
substrate have almost run out. One trick to forming large sclerotia is to
inoculate morel mycelium on a nutrient-poor substrate (such as soil) and allow
it to use its limited nutrient reserves to reach a nearby nutrient-rich
substrate. The nutrients are then translocated back into the old
mycelia where the sclerotia are formed as they begin to store the nutrients as
lipids. These sclerotia can often be quite large and have very much
the same consistency and slippery feel as walnuts. When the sclerotia are
mature, the nutrient source is removed, and water is "percolated" between the
sclerotia in the soil, perhaps simulating spring rains. After 10-12 days,
small primordia appear, and, if the conditions are correct, the morels mature
in 12-15 days. On the surface it seems like a very simple process, but as with
all mushrooms, there are many points at which the grower can make mistakes and
lose the entire crop.
The patents themselves (see also Volk and Leonard, 1989b) describe a
process for formation of sclerotia that are competent to produce fruiting
bodies. Morel sclerotia do not normally form until the nutrients of a
substrate have almost run out. One trick to forming large sclerotia is to
inoculate morel mycelium on a nutrient-poor substrate (such as soil) and allow
it to use its limited nutrient reserves to reach a nearby nutrient-rich
substrate. The nutrients are then translocated back into the old
mycelia where the sclerotia are formed as they begin to store the nutrients as
lipids. These sclerotia can often be quite large and have very much
the same consistency and slippery feel as walnuts. When the sclerotia are
mature, the nutrient source is removed, and water is "percolated" between the
sclerotia in the soil, perhaps simulating spring rains. After 10-12 days,
small primordia appear, and, if the conditions are correct, the morels mature
in 12-15 days. On the surface it seems like a very simple process, but as with
all mushrooms, there are many points at which the grower can make mistakes and
lose the entire crop.
As far as species go, morel taxonomy is a big mess. In the midwest we have what I (and most people) believe are just three species of morels:
 Morchella esculenta,
the gray morel, which is probably the same as M.crassipes, pictured at the top of this page.
Morchella esculenta,
the gray morel, which is probably the same as M.crassipes, pictured at the top of this page.
 Morchella conica, the black morel.
Morchella conica, the black morel.
 Morchella semilibera, the half-free morel.
Morchella semilibera, the half-free morel.
 I was very fortunate to visit Meramec State Park in
Missouri the weekend of April 27, 1996 to participate in the Missouri Mycological Society's MOREL MADNESS foray,
at the invitation of Ken Gilberg and Jim Winn. It was a lot of fun, and I recommend this foray very highly.
The picture is of the morel king (who found 95+ morels) the morel queen (who found 70+ morels), the morel prince (who found the largest morel),
and last year's morel king, holding the 3.75 lb. Gyromitra caroliniana he found. Most of the morels were found on south slopes, mostly under
recently dead elm trees, but also under living ash and oak trees.
I was very fortunate to visit Meramec State Park in
Missouri the weekend of April 27, 1996 to participate in the Missouri Mycological Society's MOREL MADNESS foray,
at the invitation of Ken Gilberg and Jim Winn. It was a lot of fun, and I recommend this foray very highly.
The picture is of the morel king (who found 95+ morels) the morel queen (who found 70+ morels), the morel prince (who found the largest morel),
and last year's morel king, holding the 3.75 lb. Gyromitra caroliniana he found. Most of the morels were found on south slopes, mostly under
recently dead elm trees, but also under living ash and oak trees.
REFERENCES
Leonard, Thomas J. and Thomas J. Volk, 1992. Production of new edible mushrooms in North America: shiitake and morels. in Frontiers in Industrial Mycology. Gary F. Leatham editor. Chapman Hall. p. 1-23
Ower, R., 1982. Notes on the development of the morel ascocarp. Mycologia 74: 142-144.
Ower, R., G. Mills, and J. Malachowski, 1986. Cultivation of Morchella. U.S. Patent No. 4,594,809.
Ower, R., G. Mills, and J. Malachowski, 1988. Cultivation of Morchella. U.S. Patent No. 4,757,640.
Volk, Thomas J. and Thomas J. Leonard, 1989a. Experimental studies on the morel. I. Heterokaryon formation between monoascosporous isolates of Morchella. Mycologia 81: 523-531.
Volk, Thomas J. and Thomas J. Leonard, 1989b. Physiological and environmental studies of sclerotium formation and maturation in Morchella. Appl. Env. Microbiol. 55: 3095-3100.
Volk, Thomas J. and Thomas J. Leonard, 1990. Cytology of the Life cycle of Morchella. Mycological Research 94: 399-406.
This page and other pages are © Copyright 2000 by Thomas J. Volk, University of Wisconsin-La Crosse.
Return to Tom Volk's Fungi Home Page